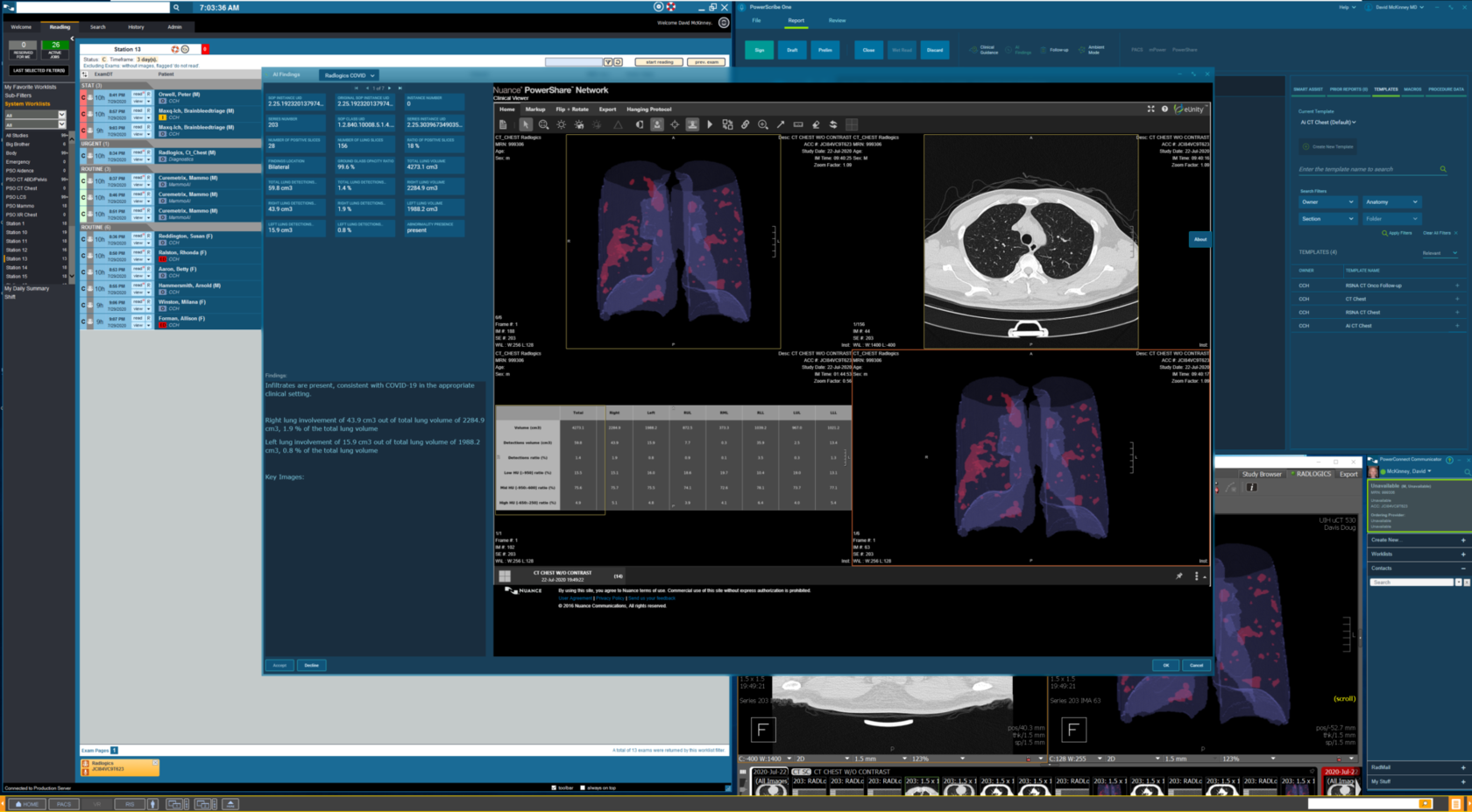
Even before the COVID-19 pandemic, increasing workloads and exponential imaging complexity not only contributed to radiologist burnout, but also caused delays in diagnosis and critical time-to-treatment. In radiology, particularly in ED and ICU settings, minutes matter. Automating and improving diagnostic imaging with cloud-based technology and the power of machine learning can be life-saving and life-changing.
Moshe Becker, CEO and co-founder of RADLogics, shares his thoughts about the impetus behind RADLogics’ software journey and the potential impact their machine-learning based solutions will have on the speed and accuracy of radiologic imaging – during the coronavirus pandemic and beyond – potentially reducing treatment turnaround time from hours to mere minutes. He’ll discuss how access to RADLogics’ applications will help meet the growing demand in the U.S. for AI-powered solutions to support the evaluation of COVID-19 patients.
Read more @ Nuance: https://whatsnext.nuance.com/healthcare/radlogics-ai-powered-solution-supports-the-evaluation-of-covid-19-patients